Calorimeters
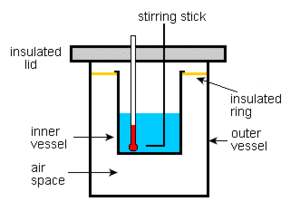
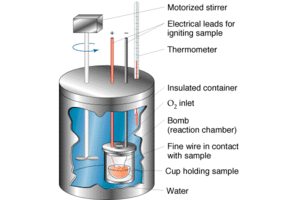
Calorimeter is an object used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. Differential scanning calorimeters, isothermal microcalorimeters, titration calorimeters and accelerated rate calorimeters are among the most common types. A simple calorimeter just consists of Thermometers attached to a metal container full of water suspended above a combustion chamber. To find the enthalpy change per mole of a substance A in a reaction between two substances A and B, the substances are added to a calorimeter and the initial and final temperatures are noted. Multiplying the temperature change by the mass and specific heat capacities of the substances gives a value for the energy given off or absorbed during the reaction. Dividing the energy change by how many moles of A were present gives its enthalpy change of reaction. This method is used primarily in academic teaching as it describes the theory of calorimetry. It does not account for the heat loss through the container or the heat capacity of the thermometer and container itself. In addition, the object placed inside the calorimeter shows that the objects transferred their heat to the calorimeter and into the liquid, and the heat absorbed by the calorimeter and the liquid is equal to the heat given off by the metals.
Types of calorimeter
Electromagnetic versus hadronic
An electromagnetic calorimeter is one specifically designed to measure the energy of particles that interact primarily via the electromagnetic interaction, while a hadronic calorimeter is one designed to measure particles that interact via the strong nuclear force. The response of a calorimeter can be described in terms of the e/h ratio. This is the measure of how well a calorimeter responds to leptons or photons versus hadrons. Ideally one would want a ratio e/h~1, this condition is called compensation.
Homogeneous versus sampling
Either of the above types can be made as a sampling calorimeter, in which the material that produces the particle shower is distinct from the material that measures the deposited energy. Typically the two materials alternate. One advantage of this is that each material can be well-suited to its task; for example, a very dense material can be used to produce a shower that evolves quickly in a limited space, even if the material is unsuitable for measuring the energy deposited by the shower. A disadvantage is that some of the energy is deposited in the wrong material and is not measured; thus the total shower energy must be estimated.A homogeneous calorimeter is one in which the entire volume is sensitive and contributes a signal.Only electromagnetic calorimeters can be homogeneous.